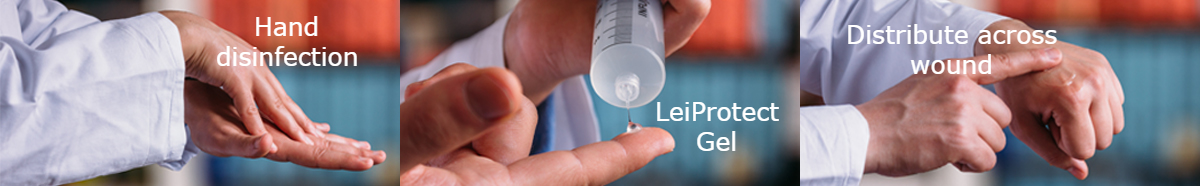
The use of LeiProtect® has been legalized on August 28th, 2017 (91.1.07-5640-S-006/16) by the Institut für Arzneimittel und Medizinprodukte (BfArM) according to § 11 of the Medical Device Regulation (MPG). It is currently used to protect leishmaniasis skin lesions from superinfections, thus promoting their healing, and to prevent female sand-flies from sucking blood in the lesions.
The use of LeiProtect® has been legalized on August 28th, 2017 (91.1.07-5640-S-006/16) by the Institut für Arzneimittel und Medizinprodukte (BfArM) according to § 11 of the Medical Device Regulation (MPG). It is currently used to protect leishmaniasis skin lesions from superinfections, thus promoting their healing, and to prevent female sand-flies from sucking blood in the lesions.
This temporary market access i.e. special approval of LeiProtect® produced by a GMP-certified original equipment manufacturer (OEM) is for the time being valid to produce 60 kg of LeiProtect®. The new European medical device regulation should come into force in 2020. This will create an application backlog for new CE certification appliers. We hope to overcome this problem, as in July 2019, our NGO has been granted a second prolongation of the temporary market access of LeiProtect® by BfArM until September 2020.
There are no Medical Device Regulations (MDR) in most leishmania endemic countries. However, the Health Ministries can validate CE approved medical devices and our NGO can then out-license the production of LeiProtect® to CL endemic countries for economic local production.